About us page
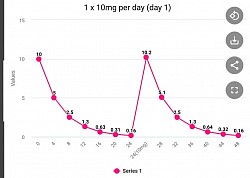
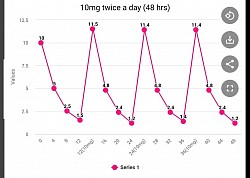
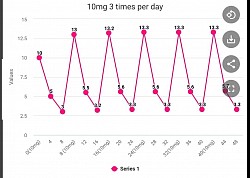
Multiple dosing per day will give better results
[Apr 2022]. Most of the clinical trials going on around the world using montelukast use the approved dosage for asthma of 10 mg once a day. Once a day dosing will likely be ineffective in these clinical trials for various medical conditions, whereas dosing multiple times a day could show good results.
The Intelgenx montelukast Alzheimers clinical trial has restarted in January 2022 after Health Canada approved higher dosing per day than the approved 10 mg per day asthma dosage. Intelgenx has decided on 30 mg twice a day (60 mg total) for one group of patients. The Emory University montelukast Alzheimers FDA clinical trial has also restarted using dosing up to 20 mg a day twice a day. Multiple times a day will give the best results for various medical conditions and have the fewest side effects.l
The following graphs are based on montelukast having a half life of 4 hours. Note that the once a day minimum value is 0.16 mg (not effective), twice a day is 1.4 mg (possibly effective), and three times a day is 3.3 mg (most effective). Emory University is dosing 20 mg twice a day with a minimum value of 2.8 and a higher average level of montelukast present in the body throughout 24 hours.
The brain's immune system
[May 2022] The following is a great overview of immune system involvement in Alzheimers. I am a follower of the concept that the robust immune system that evolved to protect us when we are young becomes overactive and off target when we grow old, and montelukast and other leukotriene blocking drugs can reduce the overactivation of the immune system in middle and old age.
Trial results by December 2022
[May 2022]. Emory University has stated in their latest postings on Clinicaltrials.gov that their completion dates will be in October and results will be announced in December 2022.
It has been a long wait because of the nearly two years delay due to Covid-19. I believe the results will be positive due to my own experience with this drug. Posted in Aug 2022
Emory montelukast Alzheimer trial
https://clinicaltrials.gov/ct2/show/NCT03991988
A tale of two trials
[Aug 2022] The Emory FDA montelukast Alzheimers trial started with 150 participants in 2019, half to be treated and half on placebos.The treated participants would take up to 20 mg twice a day for 12 months. When the trial resumed after the the Covid break, Emory cut back the trial from 150 to 32 participants, I believe, due its race with Intelgenx Medical Technologies over who will announce clinical trial results first.
Intelgenx, a Canadian company, had already started its montelukast Alzheimers trial in 2018 under Health Canada supervision, with a planned 70 participants, half which were to be treated and half to be on placebos for 26 weeks. The approved dosage was 10 mg once a day, the same as the approved dosage for asthma. Intelgenx was having problems funding the trial and the trial was halted just before the Covid break.
During the Covid break, Intelgenx secured additional funds for its trial and also received authorization from Health Canada to treat participants with up to 30 mg twice a day.
Here is what I think happened next. With the resuming of trials approaching in late 2021, Emory realized that Integenx was now in a position to complete its trial using a more effective dosage and ahead of their own. In order to come out with results first, Emory reduced its participants to 32.
As it stands now, Emory has 32 participants in its FDA double-blind placebo controlled study with treatments lasting 12 months and dosages up to 20 mg twice a day. In their last post on clinicaltrials.gov, completion is expected in October with an announcement in December 2022.
Intelgenx under the supervision of Health Canada has 70 participants in a double-blind placebo controlled study with treatments lasting 26 weeks and with dosages up to 30 mg twice a day. Their latest post on clinicaltrials.gov gave an expected completion date of October 2023. We will have to wait and see when they will announce results.
It appears that Emory will announce first and win the race in December 2022, but with only 32 participants, 16 which will receive montelukast dosages. Intelgenx will follow in late 2023 with 70 participants, 35 which will receive montelukast dosages.
Emory's trial, at 32 participants, is too small to gain FDA approval as a treatment for Alzheimers. However since montelukast is already approved as treatment for asthma, a positive outcome could encourage physicians to prescribe it off label for Alzheimers.
In the case of Intelgenx, a positive result from its larger trial could result in its version of montelukast being approved for Canadians as an Alzheimers treatment.
Emory montelukast Alzheimer trial
https://clinicaltrials.gov/ct2/show/NCT03991988
Intelgenx montelukast Alzheimers https://clinicaltrials.gov/ct2/show/NCT03402503
Leukotriene inhibiting drugs - potential benefits
[Jun 2022]. I found a great well-researched article on leukotrienes written by a PhD holder in molecular biology that explains their functions without getting into a lot of difficult medical terminology.
https://selfhacked.com/blog/leukotrienes/
Leukotrienes are lipid signaling molecules that elicits a immune response and are among the first responders in our innate immune system. There are two main types - cysteinyl leukotrienes and leukotrene B4. Montelukast inhibits cysteinyl leukotrienes by blocking them at their receptor sites on immune cells. No drug has yet been developed that safely and effectively blocks leukotriene B4 at its receptor.
Inhibiting cysteinyl leukotrienes with montelukast and other leukotriene inhibitors could bring positive results.
1. Lowering the activity of white blood cells (eosinophils and mast cells) for a lower immune response and less inflammation. A immune response that is too high and off target, which is often the case in older people, can increase aging and cellular damage.
2. Reducing cytokine production which results in less damage to healthy cells.
3. Reducing brain microglial swelling and over-activation which drives neuro-inflammation present in Alzheimers and other dementias.
4. Reducing blood-brain barrier leakiness, which is a characteristic of Alzheimers and dementia.
5. Reducing bone loss as we grow older.
Leukotrienes are powerful signaling molecules that increase immune system activation when we are under attack by bacteria, viruses and other agents. They have allowed humans to survive for hundreds of thousands of years in a hostile environment. But with modern medicine and public sanitation, we may not need such a powerful activation of our immune system that can increase cell damage, especially as we are older. It appears that leukotriene inhibiting drugs like montelukast and other drugs to come can slow down the over-activated immune system in older people and allow them to live longer and healthier lives.
You can find scientific references in the above mentioned "selfhacked" article.
DNA fragments and brain inflammation
[Aug 2022] This long term study at John Hopkins University found that high levels of cell-free DNA circulating in the blood stream of older individuals is associated with chronic inflammation and early signs of frailty and dementia. As cells die and rupture, DNA fragments are released into the bloodstream. The body's immune system sees these DNA fragments as foreign and something that needs to be removed, and it runs at a higher rate than it should. This results in chronic inflammation and the premature destruction and aging of tissue and organs. We may not have a way to prevent free range DNA, but I am convinced that we have a way to reduce the chronic inflammation that accompanies it. We can slow down leukotriene signaling, which increases cytokine production and chronic inflammation, by taking leukotriene inhibiting drugs such as montelukast. I think that when the Emory University montelukast Alzheimers clinical trial results are announced in December, we will see that this drug will reduce chronic inflammation and have a positive affect in treating Alzheimers.
DNA fragments https://medicalxpress.com/news/2022-10-blood-free-range-dna-early.html